THE ROLE OF AMNIOTIC MEMBRANE TRANSPLANTATION
A look at the clinical efficacy of using AMs for ocular surface disorders and their utility in primary eye care.
Human amniotic membrane (AM) is derived from the innermost of the three layers constituting the fetal membranes, and it lines the inner cavity of the placenta (Riau et al, 2010). There are two distinct, joint tissues: the amnion and the chorion. The amnion faces the fetus, while the chorion faces the uterus. Neither the amnion nor the chorion is vascularized; these tissues are metabolically active, and they constantly restructure to accommodate the growing fetus (Koob et al, 2015). Cytokines, growth factors, chemokines, and other regulatory factors are responsible for the continuous transformation of the amniotic tissues (Koob et al, 2015).
Amniotic membrane transplantation (AMT) in ophthalmic surgery was first documented by de Rötth in 1940, who used AMT to repair a conjunctival defect. It was not until the conclusion of the 20th century, when Kim and Tseng (1995) used AMT for ocular surface reconstruction in rabbits after total corneal epithelial removal, that the use of human AM gained significance in ophthalmic practice.
AmnioGraft (Bio-Tissue), introduced in 1997 as the first commercially available AM tissue in the United States, is a cryopreserved AM allograft for ophthalmic surgical management (Kabat and Sowka, 2015). Within a decade, IOP Ophthalmics introduced Ambio, which was created via an alternative preparation method that encompassed dehydration of the AM allograft (MiMedx Group Inc., 2012).
AMT with sutures compensates for the toxicity of fortified antibiotic drops while exerting antimicrobial activity, and it acts as a long-term medication delivery system (Sheha et al, 2009). AMT also can directly promote prompt epithelialization and reduce stromal inflammation and ulceration in corneal infections, such as bacterial keratitis (Kabat and Sowka, 2015). The mechanism of action of amniotic membrane therapeutic effects is poorly understood, but it is thought to involve the resident growth factors and cytokines within the amniotic tissue (Koob et al, 2015).
While the benefits of surgical AMT are to be considered with regard to reducing the period of hospitalization and improving patient compliance to treatment, the procedure may require additional high costs when performed in the operating room. The already inflamed and necrotic corneal tissue may not be amenable to holding sutures (Sheha et al, 2009).
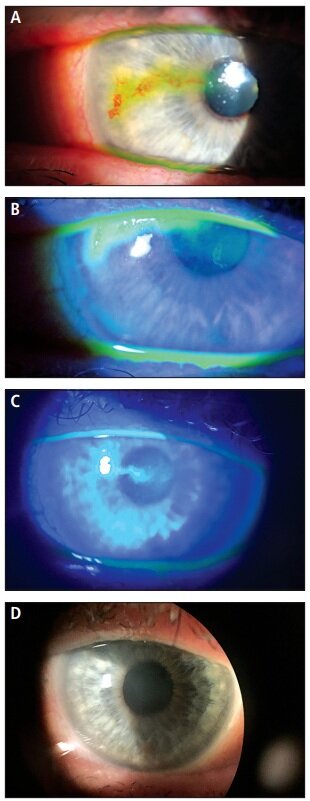
Figure 1. Corneal scarring and staining before ProKera (A and B). Five days after ProKera application (C); and two months after ProKera application (D).
So, in 2005, Bio-Tissue introduced ProKera, a single-sheet, self-retaining version of cryopreserved amniotic membrane clipped into a dual polycarbonate ring system that can be applied in-office without the use of sutures. AmbioDisk (IOP Ophthalmics) and BioDOptix (BioD, LLC), released in 2012 and 2013, respectively, are two additional sutureless AM products commercially available in the United States that are dry (IOP Ophthalmics, 2012; BioD, 2015).
AmbioDisk and BioDOptix amniotic membranes must be held in place with a contact lens applied over the membrane. This application of the AM with a bandage contact lens piggybacked on top of the AM is best performed using a speculum to hold open the palpebral aperture. The dry amniotic membranes are very delicate and should be handled with non-toothed forceps. Other manufacturers of dry membranes currently on the market inlcude Blythe Medical/Seed Biotech and Skye Biologics, but these will not be discussed further.
With the advent of ProKera, AmbioDisk, and BioDOptix, therapeutic effects of AMs are instantaneous, avoiding the necessity of sutures (Sheha et al, 2009). Although the processing, packaging, and delivery methods differ, these AM tissues are indicated for the same fundamental purpose: ocular surface rehabilitation, regeneration, renewal, and healing management. Thus, for the rest of this article, AM therapy will be generalized to the cryopreserved and dry products mentioned above.
Human Amniotic Membrane Therapy
AM use has gained a critical position in clinical management resulting from its ability to reduce ocular surface scarring and inflammation and to enhance epithelialization, while exhibiting limited immunogenicity and possessing anti-microbial properties. Histologically, the human AM is a five-layered avascular membrane averaging 0.25mm thick, with the inner surface bathed in amniotic fluid and the outer surface connecting to the chorion (Riau et al, 2010). There is an astonishing histological resemblance between the AM and the cornea and conjunctiva.
The AM epithelium rests on a basement membrane (BM) that serves as a biological scaffold, regulating epithelial morphogenesis, proliferation, and differentiation and preventing apoptosis. Beneath the BM is the substantia propria (SP) or stroma. The SP contains a compact collagenous layer, which provides tensile strength. Additionally, a fibroblastic layer—the thickest layer of the AM—consists of fibroblasts embedded in a loose network of reticulum (Riau et al, 2010). The SP forms the extracellular matrix (ECM), which is essential for growth, wound healing, and fibrosis. It also acts as a local depot, sequestering a wide range of growth factors.
The resemblance of the AM to the cornea and conjunctiva is not only anatomical, but also similar in physiologic components. Collectively, the AM contains collagen types I, III, IV, V, and VII as well as specialized proteins such as fibronectin, laminins, proteoglycans, and glycosaminoglycans (Mastrota, 2015). Another important structural component is endostatin—a BM heparan sulfate proteoglycan shown to be a potent anti-angiogenic factor—that can inhibit endothelial cell proliferation, angiogenesis, and tumor growth (Riau et al, 2010).
One of AM therapy’s proposed mechanisms of action is the release of growth factors that facilitate corneal re-epithelization and reduction of inflammation (Sheha et al, 2009). The AM contains growth factors such as epidermal growth factor (EGF), transforming growth factor (TGF), fibroblast growth factor (FGF), platelet derived growth factor (PDGF), keratinocyte growth factor (KGF), and hepatocyte growth factor (HGF) (Riau et al, 2010; He et al, 2009).
EGF is a potent mitogen for epithelial cell growth, and its high level of expression could be an explanation for ocular surface wound healing following AMT. PDGF stimulates the survival, proliferation, migration, and the deposition of ECM as well as tissue remodeling. KGF and HGF are produced both by corneal stromal keratocytes and post-AMT on the epithelial surface. It is also postulated to accelerate corneal re-epithelialization (Riau et al, 2010). TGFs stimulate synthesis and deposition of ECM proteins by increasing synthesis of protease inhibitors such as inter-alpha-inhibitor (IαI), up-regulating cell adhesion molecules, and suppressing the synthesis of matrix-degrading proteases (He et al, 2009).
A highly debated topic revolving around AMT is the role of TGF-β, which is considered to be an influential growth factor in control of fibroblast activity during corneal wound healing. Increased amounts of fibroblastic growth factors in many ocular surface disorders lead to scarring in an attempt to heal the wounds. However, the AM has been shown to suppress TGF-β signaling in keratocytes as well as in limbal and conjunctival fibroblasts (Riau et al, 2010).
Figure 2. Prokera removal.
In a study by He et al (2009), researchers reported that the abundance of high-molecular-weight (HMW) hyaluronan (HA) in the ECM of the AM exerts an anti-angiogenic and anti-inflammatory effect on the ocular surface. Additionally, HMW HA covalently links with the heavy chains (HCs) of IαI, creating an HC-HA complex that has been shown to down-regulate TGF-β promoter activation. By affecting TGF-β trafficking, the HC-HA complex is likely to be one of the active components in the AM responsible for the anti-inflammatory and anti-scarring actions that have been clinically observed in ocular surface reconstruction (He et al, 2009).
Assessment and Selection of Donor AM
From the retrieval of the donated placenta to AM transplantation, the AM collection process is multifaceted and complex. To start, disease transmission is constantly a concern and a risk factor in human organ and tissue transplantation. Therefore, comprehensive safety protocols that apply to organ transplantation also apply to AMT.
Healthy mothers may be considered eligible for voluntary placental donor status following elective cesarean section delivery (IOP Ophthalmics, 2012). The mothers provide full informed consent and are placed through extensive social habit screening as well as medical history and records review. This process screens for infectious, malignant, neurological, autoimmune, and transmissible diseases. Additionally, independent, serological Clinical Laboratory Improvement Amendments (CLIA) laboratory testing is performed and must be negative for the following: HIV-1/2, hepatitis B and C, human T-cell lymphotrophic virus (HTLV) type 1 and 2 antibodies, syphilis, cytomegalovirus, tuberculosis, and West Nile Virus (Riau et al, 2010; Bio-Tissue, 2014a; Tseng, 2009).
Donor blood is drawn two weeks prior to delivery, and if the tests yield negative results, repeat investigations are performed six months after delivery. This repeat assay is performed as a consequence of the possibility that the donor could have been in a “window period” of infection—the time between the primary infection and when it is first detectable in serum— thereby providing a false negative result on the initial tests (Riau et al, 2010). Disease transmission may also be due to inadequate aseptic preparation and procurement and to the subsequent need for expensive storage. Amniotic tissue will only be utilized if the mother and the baby go home without complications. To date, there are no known cases of AM use in medicine in which a communicable disease was transmitted.
Methods of Preservation, Sterilization, and Preparation
There are numerous methods to preserve and sterilize the AM to minimize the risk of transmitting infection. Heat- or air-dried AM loses some of its biologic properties and is not ideal for ocular surface rehabilitation (Riau et al, 2010). The AM can be lyophilized (freezedried) and can receive gamma-irradiation sterilization, which induces minimal change in its properties. The dehydrated tissue can be stored at room temperature until ocular surface treatment is necessary (Riau et al, 2010). AmbioDisk can be stored for up to five years without refrigeration.
Table 1
In the United States, two types of preservation methods are available: cryopreservation of the AM tissue (Bio-Tissue) and a dehydrated preparation (IOP Ophthalmics or BioD) (MiMedx Group, 2015; BioD, 2015; Park et al, 2008; Nguyen et al, 2014). Bio-Tissue’s propriety CryoTek process begins with the washing of the placenta using a balanced saline solution containing antibiotics such as streptomycin, penicillin, neomycin, and amphotericin B (Riau, 2010; Park et al, 2008). The AM is stored in a mixture of Dulbecco’s Modified Eagle’s Medium (DMEM) and glycerol at a ratio of 1:1, after which it can be stored in a deep-freeze at -80oC for several months (Riau et al, 2010; Park et al, 2008; Koob et al, 2014).
AM tissues from IOP Ophthalmics are dehydrated via a propriety Purion process (Mastrota, 2015). Purion processed dehydrated human amnion/chorion membrane (dHACM) allografts are initially prepared similarly to the CryoTek process by washing and sterilizing the recovered placental tissues multiple times to remove unwanted biological material (Tseng, 2009). The amnion and chorion are isolated from the placenta and then laminated to form the graft, which is then stabilized through a controlled dehydration process in which the AM is laid, stromal side down, on a suitable drying fixture (IOP Ophthalmics, 2015).
During the dehydration process, an “IOP” orientation embossment is incorporated into each graft to ensure proper placement during AMT (IOP Ophthalmics, 2015; Omni Eye Services, 2014). The “IOP” represents the epithelial surface and is turned over and carefully placed with non-toothed forceps directly on the corneal epithelium so that the “IOP” looks like “6OI.” It is tamped down and flattened with a surgical spear (Weck-Cel sponge or other sterile pledget). The cornea is dried with the surgical spear in preparation for placement. The allograft can be stored at a controlled room temperature and expires in approximately five years (Omni Eye Services, 2014; Sowka et al, 2015).
BioD also employs a dehydrated preparation method with its AM tissues using a patent-pending DryFlex process to optimize the handling characteristics of the AM (BioD, 2015). This allograft can also be stored at ambient temperatures prior to use and has a shelf life of two years (Omni Eye Services, 2014; Sowka et al, 2015).
The corneal application process is similar to that for AmbioDisk, followed by a bandage contact lens to secure the AM in place. It, too, must be smoothed out over the AM, but small wrinkles in the AM are of no consequence to the healing process. Alternatively, the practitioner may place the AmbioDisk in the center of a bandage contact lens, then apply pressure with a sterile pledget to center the AmbioDisk within the contact lens. Then, the practitioner places the bandage lens/ AmbioDisk combination directly on the cornea. The cornea may be anesthetized before application.
A recent study by Cooke et al (2014) compared cryopreserved AM and dehydrated AM to evaluate how the different processing methods affected the structural integrity and biological composition of key signaling molecules within the AM. Through bio-chemical and functional assays, they concluded that cryopreservation retains the delicate native architecture of the AM, and dehydration dramatically compacts the tissue, suggesting alteration of critical components within. Cryopreservation maintains the quantity and activity of key signaling proteins, specifically HC-HA complexes. In contrast, dehydrated tissues almost completely lacked these crucial components.
Figure 3. Superficial punctate keratitis in a severe dry eye patient (A) and the same patient after AmbioDisk use (B).
Previously, however, Allen et al (2013) conducted an experiment utilizing electron microscopy to assess the visual and structural comparisons between cryopreserved AM and dehydrated AM. The group reported that dehydrated AM has enhanced structural properties and biochemical stability and is a superior substrate to conventional cryopreserved AM.
The current literature comparing the different AM preparations and their respective capability to retain both the integrity of the AM and its therapeutic components is scarce and capricious. It is evident that the large variability of results in these studies necessitates future research to adequately determine whether an optimal method for AM preparation exists.
Indications for Amniotic Membrane Therapy
In the past 20 years, numerous studies have been published on the therapeutic effects of AM therapy, and the indications for AMT have expanded as a result. AM therapy is now widely used in various ocular manifestations for treatment and management. Many indications for AM therapy, both surgical and sutureless, are documented in the current literature. Table 1 shows a non-exhaustive sampling of those indications (Riau et al, 2010; He et al, 2009; Nguyen et al, 2014; Koob et al, 2014; and others. Full list available at www.clspectrum.com/references.)
Research Examples
In a study by Park et al (2008), 62 eyes from 58 patients who had undergone surgical AMT for various ocular surface diseases were investigated for the efficacy of AMT in treating those diseases. The success rate in patients who had neurotrophic ulcer, inflammatory corneal ulcer, scleral ulcer, and bullous keratopathy were 93.3%, 66.7%, 92.9%, and 100%, respectively. The researchers concluded that AMT has a high success rate in the treatment of these conditions and with a low rate of complications.
A 2009 study by Sheha et al reported their experience using ProKera in three consecutive cases of severe microbial keratitis. On the first day after application, all three patients reported significant pain relief. Patients 1 and 2, who both had staphylococcal keratitis for two weeks that was unresponsive to antibiotic therapy, presented with paracentral corneal ulcers in their left eyes with surrounding stromal haze and hypopyon. After 14 days of ProKera, the corneal surface was completely healed in both cases.
Patient 3, who presented with Pseudomonas keratitis for two months, demonstrated severe pain and a large 9.0mm corneal epithelial defect with necrotic inflammation and 360o of corneal neovascularization. On the second day after ProKera application, the pain had disappeared, and the epithelial defect and inflammation were decreased. By day 10, the epithelial healing did not progress as a result of inadequate apposition of the device to the cornea resulting from lower lid ectropion. Therefore, AMT with sutures was used, and after eight days, the cornea was completely healed.
The authors concluded that although future clinical trials with larger sample sizes are required, ProKera is an effective regimen for the treatment of microbial keratitis.
Kheirkhah, Johnson et al (2008) evaluated five eyes in five patients who had acute alkaline burns for the clinical outcomes of sutureless AMT use within three days of the incident. After AMT, the conjunctival defects re-epithelialized in eight days, while limbal and corneal defects healed in 14 days. During a year of monthly follow-up visits, all eyes retained a stable surface with improved corneal clarity, without limbal deficiency or symblepharon. One of the indications of AMT is to prevent tissue adhesion, which can be beneficial during ocular surface treatment (Young et al, 1991).
The experiment yielded the conclusion that sutureless AMT allows for prompt delivery of its therapeutic and biological effects, which assists in preserving remaining limbal stem cells for rapid expansion and prevents late cicatricial complications with mildto-moderate acute alkaline burns.
Stevens-Johnson syndrome (SJS) is a severe cellmediated, delayed hypersensitivity condition related directly to medications or to epithelial cell antigens modified by medication exposure (Kanski and Bowling, 2001). It affects the skin and mucous membranes of the entire body and is of great concern for both patients and practitioners (Russell, 2015). Kolomeyer et al (2013) treated a 19-year-old patient who developed acute SJS with ocular involvement after oral ingestion of antibiotics. Slit lamp examination performed two weeks later showed ocular SJS signs of severe inflammation with areas of ulceration along all four eyelids as well as complete sloughing of bulbar and palpebral conjunctivae bilaterally, including the limbus.
Three weeks after ProKera placement, slit lamp examination showed complete re-epithelialization of both corneas and conjunctivae, with only trace conjunctival injection and minor limbal epithelial irregularities. Three months post-AMT, there was an absence of signs of clinically significant scarring in either eye, and the visual acuity (VA) was 20/20. Fourteen months post-procedure, VA remained stable, and the patient did not have dry eye, photophobia, clinically significant scarring, or symblepharon.
The researchers concluded that ProKera placement may be applicable for the treatment of ocular surface manifestations of acute SJS, particularly in patients followed in an outpatient setting with milder forms of disease.
A study published in 2014 evaluated and compared the indications and outcomes of ProKera versus AmbioDisk in the management of various ocular surface disorders in 22 eyes of 20 patients. Giannikas et al (2014) completed a retrospective chart review of all of the patients who received AMT between 2010 and 2013 at a tertiary medical center’s cornea practice.
During that time period, 64% of the eyes were treated with ProKera, and 36% were treated with AmbioDisk. The indications for AMT included, but were not limited to, non-healing infectious corneal ulcers (ProKera 21%, AmbioDisk 13%), neurotrophic keratopathy (ProKera 43%, AmbioDisk 25%), and non-healing epithelial defects over previous corneal transplants (ProKera 14%, AmbioDisk 63%).
In the study, complete success with AMT was defined as complete healing of the ocular surface without necessitating further intervention; partial success was defined as incomplete healing requiring further intervention (bandage contact lens, tarsorrhaphy, conjunctival flap); and treatment failure was defined as lack of improvement with transplant or premature discontinuation of AMT prior to improvement. Overall success—complete and partial—was demonstrated in 68% of eyes, with ProKera treatment accounting for 64% of those successes and AmbioDisk accounting for 75%.
The results showed that both modalities had similar success rates, minimal incidence of deleterious side effects, and there was no statistically significant difference in their performance across multiple treatment indications. The authors concluded that ProKera and AmbioDisk are both successful in promoting healing in a variety of ocular surface disorders.
Design and Clinical Procedure
ProKera
ProKera is obtainable in three different designs, with each designated for different indications pertaining to severity. ProKera Slim is 100μm thick, with a lower profile and comfort ring design to improve patient comfort. It is recommended for mildto-moderate indications such as microbial or herpes simplex keratitis, corneal abrasions, and recurrent corneal erosions (Russell, 2015). The original ProKera, introduced in 2005, is also 100μm thick and is recommended for moderate-to-severe conditions such as neurotrophic ulcers, severe infectious keratitis, corneal wounds, and corneal transplantations with high-risk of graft rejection (Russell, 2015; Bio-Tissue, 2014b). Figure 1 shows an example of the effect that ProKera can have, in which a female patient had a previous diagnosis of herpes simplex that left her cornea scarred and irregular. One of the authors fit her with ProKera. After two months, there was a total absence of scarring.
The most recent design, ProKera Plus, has multiple layers of amniotic membrane, providing longer biologic and healing properties on the ocular surface. It is 200μm thick and is endorsed for severe indications, such as chemical and thermal burns, SJS, severe corneal ulcers, and severe corneal wounds.
ProKera consists of a piece of AmnioGraft clipped into a dual polymethyl methacrylate (PMMA) symblepharon ring system (Park et al, 2008). The device is manufactured so that the AM tissue is positioned with the epithelial side up. Consequently, the stromal side of the AM tissue is in contact with the corneal surface and fits snugly between the cornea and the eyelids by conforming to the corneal surface like a contact lens (Sheha et al, 2009; Park et al, 2008). As a result of recent improvements in application, ProKera is relatively simple to offer in a clinical office setting using aseptic technique.
Because ProKera arrives maintained in an antiinfective storage medium, it must be prepped for ocular placement by completely rinsing the device with sterile saline (Thimons, 2014). The ProKera must be thoroughly rinsed on both sides due to the glycerol media; it is stored in glycerol to prevent freezing. Failure to thoroughly rinse the ProKera will result in a stinging, burning sensation immediately after applying to the patient’s eye.
After rinsing, the clinician must don gloves and carefully grab the ring with the fingers or with sterile, blunt instruments. Once anesthetic eye drops are administered, the clinician holds the upper eyelid and instructs the patient to look down as ProKera is applied in the superior fornix and slid down under the lower lid (Park et al, 2008). To achieve comfortable ring placement, a partial tape-tarsorrhaphy (or tapesorrhaphy) is used to tape the temporal half of the eyelid closed to decrease movement, foreign body sensation, and blink-induced discomfort (Thimons, 2015; Groves, 2014; Mangan, 2014). Partially closing the eyelid maintains a small opening nasally so that any necessary topical medical therapy can be applied while maintaining patient comfort.
ProKera is FDA-approved to remain on the eye for a maximum of eight weeks after application, until the ocular surface has healed or the tissue has dissolved (Park et al, 2008; Groves, 2014). However, healing is typically complete between one and two weeks, and ProKera subsequently can be removed using blunt, sterile forceps (Figure 2). Commonly, the ProKera membrane is totally absorbed by the cornea in less than a week, at which time the plastic ring may be removed and discarded.
AmbioDisk
AmbioDisk, a sutureless, overlay AM graft, is IOP Ophthalmics’ fourth-generation AM technology (IOP Opthalmics, 2012). The 15.0mm configuration is available in both the Ambio2 and Ambio5 with a new 9.0mm and 12.0mm diameter version available in the Ambio2 option only (IOP Ophthalmics, 2015; Omni Eye Services, 2014; Sowka et al, 2015). Ambio2 is 35μm thick and is conventionally used for mild and moderate indications, such as nonhealing epithelial defects, neurotrophic ulcerations, corneal erosions, acute corneal burns, and post-infectious keratitis (Figure 3). Ambio5 has a 100μm thickness and is recommended for more severe corneal compromise that would demand a more aggressive therapy (IOP Ophthalmics, 2012).
AmbioDisk is packaged in a double peel-pouch packaging configuration with the stromal matrix in contact with the metallic side of the pouch (IOP Ophthalmics, 2015). The BM, facing away from the metallic side of the pouch, can be easily identified by the “IOP” lettering embossed on the center of the graft (MiMedx Group, 2015; IOP Ophthalmics, 2015). If necessary, prior to placement, the AmbioDisk may be trimmed in its dry state to a different size for more appropriate fitting (IOP Ophthalmics, 2015).
A speculum is positioned to retract the eyelids, and the dry AmbioDisk is placed on a dry ocular surface with the “IOP” oriented toward the cornea (IOP Ophthalmics, 2015). A Kontur Precision spherical contact lens that comes with the 15.0mm AM is then placed over the graft to bandage the AmbioDisk to the ocular surface (Omni Eye Services, 2014; Sowka et al, 2015). The 9.0mm and 12.0mm AmbioDisk does not include a contact lens, so practitioners will need to supply the bandage lens when using these diameters.
BioDOptix
The BioDOptix AM is also a dehydrated tissue that is available in both circular and rectangular configurations with central thicknesses of 40μm to 60μm. It may be easily trimmed prior to placement for optimal fitting (Mangan, 2014). The circular and rectangular BioDOptix configurations are both obtainable in two sizes with 12.0mm or 15.0mm discs and 1.5cm2 x 2.0cm2 or 2.0cm2 x 3.0cm2 rectangular designs (BioD, 2015).
Owing to its dehydrated state, application of BioDOptix is comparable to that with AmbioDisk; it is applied to a dry cornea after placing an eyelid speculum and smoothing the disc and bandage contact lens with a Weck-Cel sponge (Figure 4).
Coding and Billing
Practitioners who perform AMT in the office should use the Current Procedural Terminology (CPT) code 65778 when submitting this service for reimbursement (Omni Eye Services, 2014; Sowka et al, 2015; Rumpakis, 2015). As of Jan. 1, 2014, the American Medical Association defines CPT code 65778 as: “Placement of amniotic membrane on the ocular surface; without sutures.” This service code entails a 10-day global period in which office visits during this time will not be reimbursed by insurance. However, at the time that this article was going to press, the removal of this 10- day period was being considered. (Omni Eye Services, 2014; Rumpakis, 2015).
Additionally, the Centers for Medicare & Medicaid Services (CMS) has authorized payment policies for the procedure to be performed in both facility and non-facility settings, and all local Medicare carriers have established coverage polices for this procedure (Rumpakis, 2015). However, regarding CMS, a separate charge and reimbursement for the supply of the AM is not allowed (Corcoran Consulting Group, 2013). Reimbursement for the supply is included with payment for the procedure. It is important to note that when the surgical code is used for AM placement, an office visit code may not be used because the office visit is considered content of service.
Other commercial carriers do not necessarily follow the CMS approach and may use policies that allow for reimbursement of the procedure and the materials separately. If the payment policy exists, the appropriate Healthcare Common Procedure Coding System (HCPCS) Level II code is V2790, which is defined as: “Amniotic membrane for surgical reconstruction, per procedure.” (Omni Eye Services, 2014; Sowka et al, 2015; Rumpakis, 2015).
Conclusion
It is apparent that AMT has inspired a paradigm shift in clinical treatment and management of ocular surface disorders and diseases. The intricacies of an AM act as a BM, possessing structural components like collagen that support the growth of ocular surface epithelium and facilitate epithelial cell migration. The AM stroma recruits growth factors and proteins that provide a non-inflammatory microenvironment for the ocular surface to regenerate and heal. This provides a vital signaling pathway for the facilitation of corneal stroma, conjunctival stroma, and limbal stem cell communication (Koob et al, 2014).
Sutureless AM therapy allows for an expanded optometric scope to regeneratively heal patients who are suffering from a host of painful and sight-threatening conditions. This powerful technology is now ready and available for the primary eyecare optometrist to use without necessitating referral to a tertiary corneal specialist. CLS